Thanks to medical and scientific progress over the past decades, many people with cancer are living longer than ever before. This is possible because treatments continuously evolve, with each scientific discovery opening avenues for more effective and targeted therapies, further driving progress in cancer management.
Early approaches to cancer treatment have been documented for centuries, with surgery and hormone therapy being among the earliest recorded methods.1 This was followed by the introduction of radiation therapy in the late 19th century and chemotherapy in the 1940s.1 Traditional treatments like chemotherapy and radiation act rapidly, affecting both cancerous and healthy tissues. As a result, more targeted cancer therapies are needed to specifically target cancer cells whilst reducing the risk of damage to healthy tissues, improving the likelihood of favourable treatment outcomes.
At Daiichi Sankyo Europe, we focus on antibody-drug conjugate (ADC) technology, a therapy that delivers medicine directly to tumour cells.
Advancing Modern ADC Technology
ADCs target tumour-specific antigens – enabling a more precise delivery of medicine directly to cancer cells. Thanks to this approach, ADCs offer an opportunity by targeting cancer cells while affecting healthy cells and tissues less.
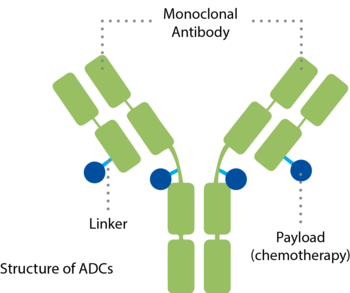
ADCs combine a specific antibody and a potent payload in a single molecule.5,6 They consist of three components:
- An antibody that binds to a specific target, uniquely found on the surface of cancer cells – a mechanism similar to a key that fits only into one lock
- A medicine, also called cytotoxic payload, with the ability to inactivate the cancer cell
- And a linker that connects both components
Each part plays a critical role in delivery and efficacy. Current and ongoing ADC research focuses on improving the targeting antibodies, finding different target antibodies and targets, ensuring controllable linker resolution in the right environment and adding multiple payloads. Such investigations may allow for broader applicability across many tumour types.2,3,4
The History of ADCs
ADCs have a rich history dating back to the early 1900s when Paul Ehrlich first proposed the concept of targeted drug delivery.3 However, it wasn't until the 1960s that the first documented in vitro studies exploring ADCs took place.7 The field advanced significantly in the 1980s and in 2000 the U.S. Food and Drug Administration (FDA) approved the first ADC for treating acute myeloid leukaemia (AML). Although it was initially withdrawn in 2010 due to toxicity concerns, it was reapproved in 2017 with adjusted dosing.8 Since then, the ADC landscape has evolved rapidly, with 14 ADCs approved worldwide in 2024 and over 150 ongoing clinical trials, marking a new era in targeted cancer therapy.9
The Future of ADCs
The interest and optimism surrounding ADCs are evident from the surge of clinical trials, which have increased from 50 in 2013 to nearly 300 in 2023 alone. Currently, 14 ADCs are globally approved across various cancer indications, with 17 in late-stage Phase 3 trials and three in Phase 4 trials.10,11
Looking ahead, at Daiichi Sankyo Europe, we envision a future where we can discover new opportunities for treating cancer, further enhance our precision technologies, and design new payloads specifically for ADCs. Our ambition is to bring ADCs to patients at earlier stages of their treatment journey.
We are committed to advancing ADC-Technology, leveraging our unique position to continue addressing challenges and unlocking the full potential of this innovative cancer treatment.
Research and Development in Oncology
Daiichi Sankyo Europe is committed to researching and developing innovative therapies for patients. Our European headquarters in Munich, Germany, serves as a hub for key operations spanning multiple sites.
The Production and Research Centre Pfaffenhofen

Our state-of-the-art production facilities in Pfaffenhofen, Germany, serve as a key manufacturing and distribution hub, supplying products to over 50 countries worldwide. By 2030, we plan to invest around 1 billion euros, expanding Pfaffenhofen into a global innovation centre, thereby reinforcing our commitment to advancing pharmaceutical development and manufacturing in Europe.
Furthermore, research on human tissue is increasingly important for understanding how the human body manages the effects of new medications. Our Translational Research Center Europe in Martinsried, near Munich, is at the forefront of this research, helping us find treatment options with fewer side effects.
References
- American Cancer Society. The History of Cancer [Accessed May 2025]
- Cheng-Sánchez I, et al. Marine Drugs. 2022;20(8)
- Fu Z, et al. Signal Transduct Target Ther. 2022;7(1)
- Conilh L, et al., J. Hematol. Oncol. 2023;16(3)
- Trail P.A, et al. Pharmacol Ther. 2018 ;181
- Tsuchikama K, An Z. Protein Cell. 2018;9(1)
- Sau S, et al. Drug Discovery Today. 2017;22(10)
- Liu K, et al. Molecular Cancer. 2024:23(62)
- Dreps A. Applied Clinical trials. 2024
- Tsuchikama K, et al. Nature Reviews Clinical Oncology. 2024;21(203-223)
- Shastry M, et al. ASCO Educational Book. 2023;43


